on
19++ Partition coefficient of iodine ideas in 2021
Partition Coefficient Of Iodine. The partition coefficient increases with increase in concentration of solute. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. Where K d is now specifically the distribution coefficient or molar concentration ratio of. Ii The aqueous layer is separated and shaken with 50 cm3 of the pure organic solvent.
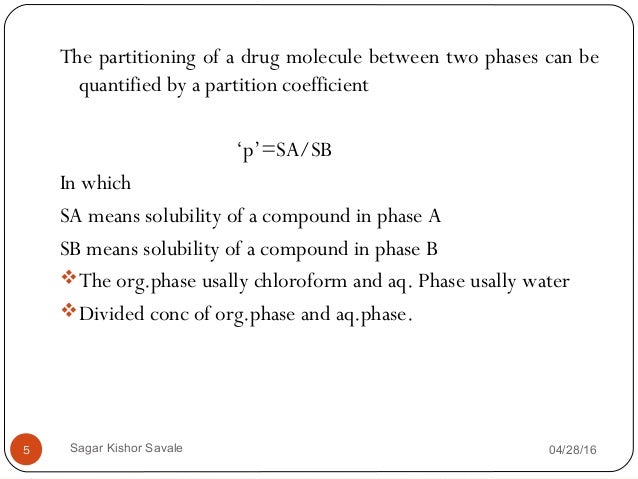 Partition Coefficient From slideshare.net
Partition Coefficient From slideshare.net
Wilhelm Office of Radiation and Indoor Air US. Where K d is now specifically the distribution coefficient or molar concentration ratio of. The distribution coefficient for the partition of molecular iodine between water and carbon tetrachloride is 85. Transactions of the Korean Nuclear Society Spring Meeting Jeju Korea May 23-24 2019 3. Tion coefficient KRe as developed in the previous paper equation 44 Weber et al 1986. Conclusions Molecular iodine concentrations and partition.
Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide.
Where K d is now specifically the distribution coefficient or molar concentration ratio of. For conditions of equilibrium between two immis-. Wilhelm Office of Radiation and Indoor Air US. Decomposition of Iodine molecule Indeterminate error PH and temperature as a factor CONCLUSION The partition coefficient was 0736 The formation constant for the tri-iodide ion was found to be 250 REFERENCES 1. The molecular phase of iodine is similar to that of I2 in both carbon tetrachloride and water. Partition coefficient is also known to be distribution coefficient.
 Source: researchgate.net
Source: researchgate.net
Environmental Protection Agency Washington DC. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. The partition coefficient Kp quantifies the equi- librium partitioning of a liquid or supercooled organic solute between two phases K– cC where Ci and C are the molar concentrations of solute i in phases x and y respectively. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. The distribution coefficient for the partition of molecular iodine between water and carbon tetrachloride is 85.
 Source: researchgate.net
Source: researchgate.net
In this experiment the partition coefficient of iodine - K D I 2 - was determined by placing water and iodine dissolved in iso-octane in a separating funnel mixing both solvents thoroughly and by then separating the phases from each other. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. I just completed a chemistry practical where I had to determine the partition coefficient of iodine between KI solution and n-hexane. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. Three solubility models which alternatively utilize both octanolwater partition coefficients and HPRPLC retention times as predictors of the saturation-limit aqueous activity coefficient will also be compared.
 Source: in.pinterest.com
Source: in.pinterest.com
For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. I just completed a chemistry practical where I had to determine the partition coefficient of iodine between KI solution and n-hexane. Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. For conditions of equilibrium between two immis-. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide.
 Source: researchgate.net
Source: researchgate.net
Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. It is the distribution of solute such as iodine between the solvents carbon tetrachloride and water. The partition coefficient Kp quantifies the equi- librium partitioning of a liquid or supercooled organic solute between two phases K– cC where Ci and C are the molar concentrations of solute i in phases x and y respectively. Environmental Protection Agency Washington DC.
 Source: researchgate.net
Source: researchgate.net
Calculate the partition coefficient for iodine between the organic solvent and water. Environmental Protection Agency Washington DC. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. Transactions of the Korean Nuclear Society Spring Meeting Jeju Korea May 23-24 2019 3. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O.
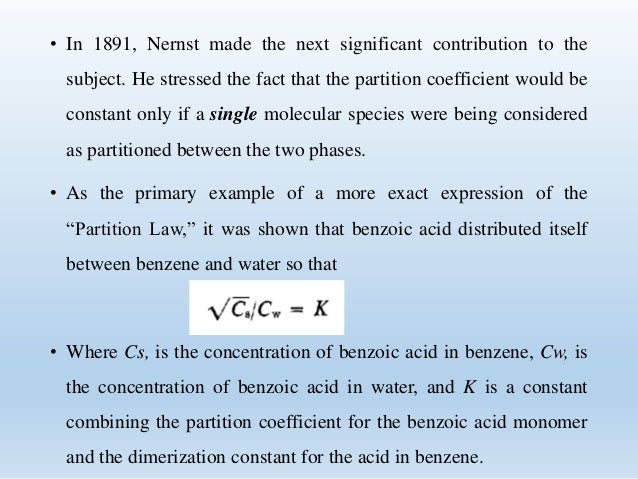 Source: slideshare.net
Source: slideshare.net
For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. 50 mL of 0010 M aqueous solution of I2 iodine subscript 2is extracted with 50. I just completed a chemistry practical where I had to determine the partition coefficient of iodine between KI solution and n-hexane. I searched online and I only found experiments where they used different solvents. Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No.
 Source: slideshare.net
Source: slideshare.net
Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. Partition coefficient of iodine in various pH solutions under radiation. Concentration of iodine in the water layer is 40 x 10 3 mol dm 3 and in the organic solvent is 10 x 10 2 mol dm 3. The distribution coefficient for the partition of molecular iodine between water and carbon tetrachloride is 85. PARTITION COEFFICIENT K d VALUES Volume III.
 Source: researchgate.net
Source: researchgate.net
50 mL of 0010 M aqueous solution of I2 iodine subscript 2is extracted with 50. Decomposition of Iodine molecule Indeterminate error PH and temperature as a factor CONCLUSION The partition coefficient was 0736 The formation constant for the tri-iodide ion was found to be 250 REFERENCES 1. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. I calculated a K 086 and I wanted to compare it to the true value and reference it in my lab report.
 Source: slideplayer.com
Source: slideplayer.com
Partition coefficient is also known to be distribution coefficient. Environmental Protection Agency Washington DC. Decomposition of Iodine molecule Indeterminate error PH and temperature as a factor CONCLUSION The partition coefficient was 0736 The formation constant for the tri-iodide ion was found to be 250 REFERENCES 1. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool.
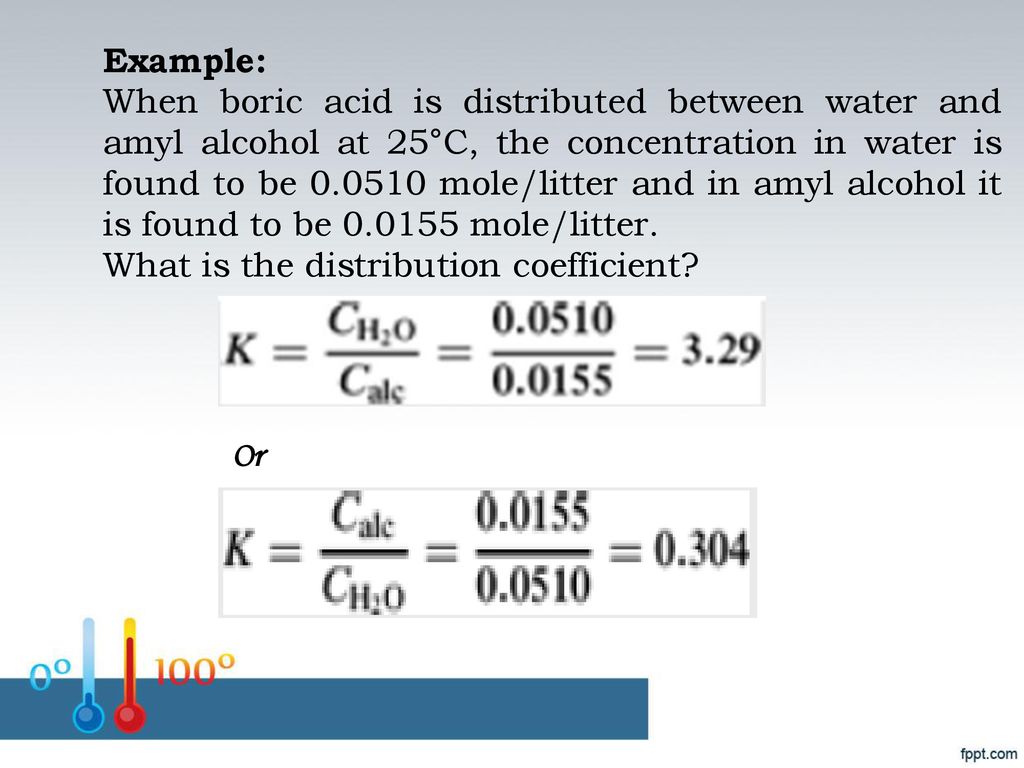 Source: slideplayer.com
Source: slideplayer.com
Ii The aqueous layer is separated and shaken with 50 cm3 of the pure organic solvent. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. Environmental Protection Agency Washington DC. Where K d is now specifically the distribution coefficient or molar concentration ratio of. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O.
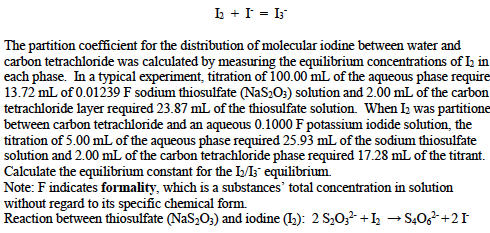 Source: chegg.com
Source: chegg.com
The distribution coefficient for the partition of molecular iodine between water and carbon tetrachloride is 85. Wilhelm Office of Radiation and Indoor Air US. K d is an equilibrium constant called the distribution coefficient. Transactions of the Korean Nuclear Society Spring Meeting Jeju Korea May 23-24 2019 3. The partition coefficient Kp quantifies the equi- librium partitioning of a liquid or supercooled organic solute between two phases K– cC where Ci and C are the molar concentrations of solute i in phases x and y respectively.
 Source: slidetodoc.com
Source: slidetodoc.com
The partition coefficient increases with increase in concentration of solute. The partition coefficient increases with increase in concentration of solute. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. Environmental Protection Agency Washington DC. Partition coefficient of iodine in various pH solutions under radiation.
 Source: researchgate.net
Source: researchgate.net
Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. DW89937220-01-7 Project Officer Ronald G. Concentration of iodine in the water layer is 40 x 10 3 mol dm 3 and in the organic solvent is 10 x 10 2 mol dm 3. Where K d is now specifically the distribution coefficient or molar concentration ratio of. I just completed a chemistry practical where I had to determine the partition coefficient of iodine between KI solution and n-hexane.
 Source: slideshare.net
Source: slideshare.net
I calculated a K 086 and I wanted to compare it to the true value and reference it in my lab report. The partition coefficient increases with increase in concentration of solute. Environmental Protection Agency Washington DC. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide. DW89937220-01-7 Project Officer Ronald G.
 Source: slideshare.net
Source: slideshare.net
I just completed a chemistry practical where I had to determine the partition coefficient of iodine between KI solution and n-hexane. Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. DW89937220-01-7 Project Officer Ronald G. Concentration of iodine in the water layer is 40 x 10 3 mol dm 3 and in the organic solvent is 10 x 10 2 mol dm 3.
 Source: researchgate.net
Source: researchgate.net
Review of Geochemistry and Available K d Values for Americium Arsenic Curium Iodine Neptunium Radium and Technetium July 2004 Interagency Agreement No. The distribution coefficient for the partition of molecular iodine between water and carbon tetrachloride is 85. Three solubility models which alternatively utilize both octanolwater partition coefficients and HPRPLC retention times as predictors of the saturation-limit aqueous activity coefficient will also be compared. Transactions of the Korean Nuclear Society Spring Meeting Jeju Korea May 23-24 2019 3. Ii The aqueous layer is separated and shaken with 50 cm3 of the pure organic solvent.
 Source: researchgate.net
Source: researchgate.net
It is the distribution of solute such as iodine between the solvents carbon tetrachloride and water. Concentration of iodine in the water layer is 40 x 10 3 mol dm 3 and in the organic solvent is 10 x 10 2 mol dm 3. Equilibrium partition coefficients were experimentally measured for volatile fission products of cesium and iodine between liquid sodium pool and the inert cover gas to obtain the coefficients at high temperature between 600 and 850 C and to study the dependence of the partition coefficients upon the concentration in the sodium pool. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. Iodine partition coefficients defined as the ratio of the concentration of iodine species in the aqueous solution to the iodine concentration in the vapor phase were measured at simulated PWR steam generator conditions 285C and 69 MPa using carrier-free radioactive T I in the form of sodium iodide.
 Source: chegg.com
Source: chegg.com
Where K d is now specifically the distribution coefficient or molar concentration ratio of. For the iodine-water-cyclohexane system K d 1 2 in C 6 H 12 I 2 in H 2 O. K d is an equilibrium constant called the distribution coefficient. For conditions of equilibrium between two immis-. Environmental Protection Agency Washington DC.